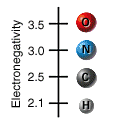
At the top right are 3 columns containing bonded pairs of atoms depicted by ball-and-stick models. A connecting stick between two atoms represents a single bond. The first column contains a bonded molecule of 2 carbon atoms and a bonded molecule of 2 hydrogen atoms one below the other. The second column contains a bonded molecule of 1 nitrogen and 1 hydrogen atoms, a bonded molecule of 1 oxygen and 1 carbon atoms and a bonded pair of 2 oxygen atoms one below the other. The third column contains a bonded molecule of 1 oxygen and 1 hydrogen atoms, a bonded molecule of 1 oxygen and 1 nitrogen atoms and a bonded molecule of 1 nitrogen and 1 carbon atoms one below the other. 4 beakers labeled as ‘Nonpolar’, ‘Moderately polar’, ‘Highly polar’ and ‘Most polar’ are arranged from left to right respectively.Pressing the spacebar key to select an item, use the Arrow keys to navigate and press the enter key to drop the selected item.












