3. Why are protons so important?
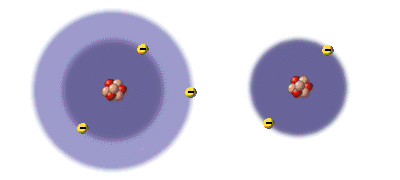
Protons determine the chemical identity of an atom because they are permanently anchored in the nucleus, where their attraction determines how many electrons the atom can hold. By contrast, electrons come and go, and their numbers vary. Despite very different chemical behavior, the atom and the ion shown below are both lithium because they have three protons in the nucleus.
Lithium(Li)
3 protons
3 electrons
Lithium(Li+)
3 protons
2 electrons